You have no items in your shopping cart.
-
Glidewell HT™ Implant Surgical Kit
70-1071-SRG0375
Product DescriptionThe Glidewell HT™ Implant Surgical Kit includes instrumentation that is machined from corrosion-resistant, surgical stainless steel, and features standard connectivity. The color-coded surgical kit clearly maps the drilling protocol... -
Glidewell HT™ Implant Guided Surgical Kit
70-1071-SRG0376
Product DescriptionThe Glidewell HT™ Implant Guided Surgical Kit includes instrumentation that is machined from high-quality, corrosion-resistant, surgical stainless steel. Color-coded bands correspond to implant diameter, matching the color of the... -
Glidewell HT™ Implant Ø3.0 x 11.5 mm
70-1189-IMP0001
Product DescriptionGlidewell HT™ Implants allow for precise control during placement, engage a maximum amount of bone, and achieve a high degree of primary stability in a wide variety of clinical... -
Glidewell HT™ Implant Ø3.0 x 13 mm
70-1189-IMP0002
Product DescriptionGlidewell HT™ Implants allow for precise control during placement, engage a maximum amount of bone, and achieve a high degree of primary stability in a wide variety of clinical... -
Glidewell HT™ Implant Ø3.0 x 16 mm
70-1189-IMP0003
Product DescriptionGlidewell HT™ Implants allow for precise control during placement, engage a maximum amount of bone, and achieve a high degree of primary stability in a wide variety of clinical... -
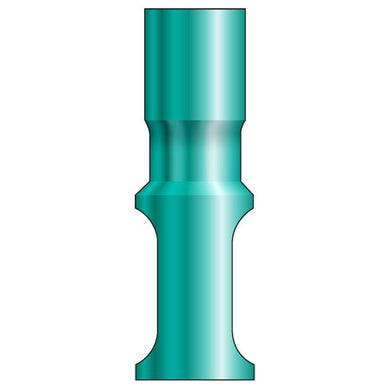
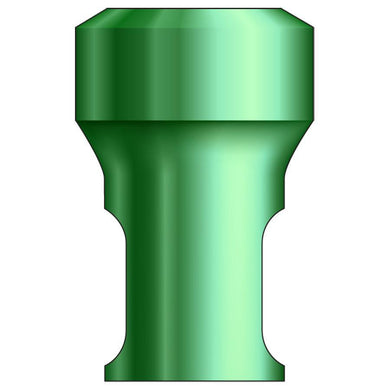
Glidewell HT™ Implant Analog - Ø3.0 Implant
70-1190-PRC0035
Product DescriptionGlidewell HT™ Implant Analogs are platform-specific replicas of dental implant fixtures, used in a working model to represent the location and platform orientation of a seated implant. They are... -
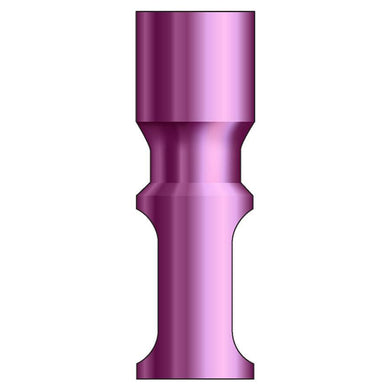
Glidewell HT™ Implant Analog - Ø3.5 Implant
70-1190-PRC0036
Product DescriptionGlidewell HT™ Implant Analogs are platform-specific replicas of dental implant fixtures, used in a working model to represent the location and platform orientation of a seated implant. They are... -
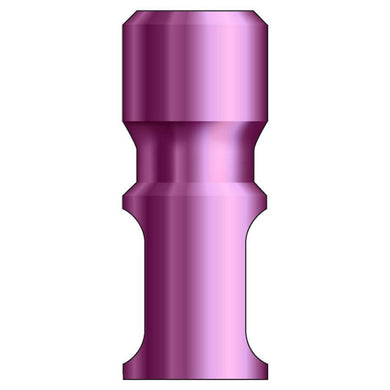
Glidewell HT™ Implant Analog - Ø4.3 Implant
70-1190-PRC0037
Product DescriptionGlidewell HT™ Implant Analogs are platform-specific replicas of dental implant fixtures, used in a working model to represent the location and platform orientation of a seated implant. They are... -
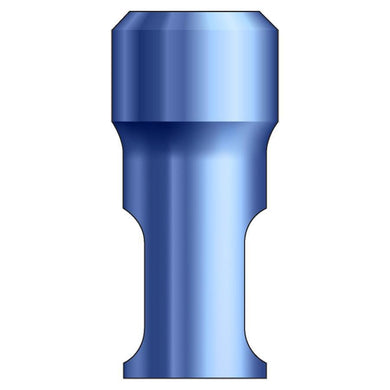
Glidewell HT™ Implant Analog - Ø5.0 Implant
70-1190-PRC0038
Product DescriptionGlidewell HT™ Implant Analogs are platform-specific replicas of dental implant fixtures, used in a working model to represent the location and platform orientation of a seated implant. They are... -
Glidewell HT™ Implant Analog - Ø7.0 Implant
70-1190-PRC0039
Product DescriptionGlidewell HT™ Implant Analogs are platform-specific replicas of dental implant fixtures, used in a working model to represent the location and platform orientation of a seated implant. They are... -
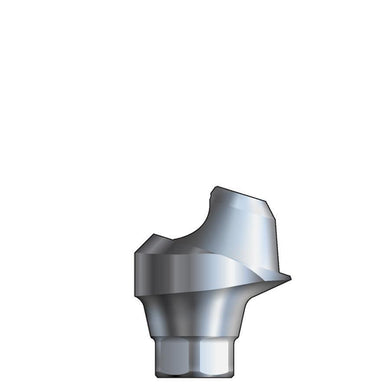
Glidewell HT™ Implant 17° Multi-Unit Abutment 2.5 mmH - Ø5.0 Implant
70-1190-PRS0002
Product DescriptionGlidewell HT™ Implant Multi-Unit Abutments are prefabricated, screw-retained intraoral abutments intended to be connected directly to endosseous implants in partially or fully edentulous patients for the retention of cast... -
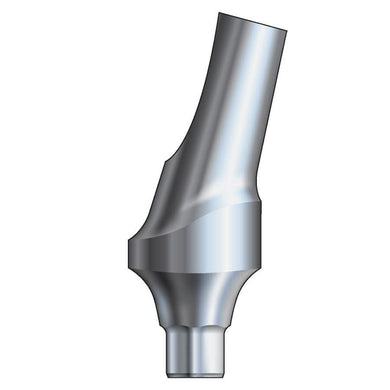
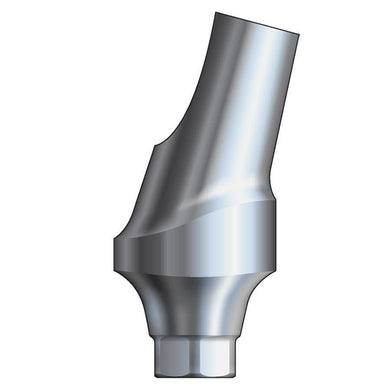
Glidewell HT™ Implant 15° Anterior Esthetic Abutment - Ø3.0 Implant
70-1190-PRA0042
Product DescriptionGlidewell HT™ Implant Esthetic Abutments are prefabricated, screw-retained intraoral abutments intended to be connected directly to an endosseous implant for retention of a cemented dental prosthesis. They may be... -
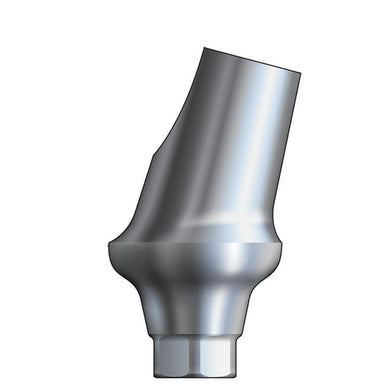
Glidewell HT™ Implant 15° Anterior Esthetic Abutment - Ø3.5/4.3 Implant
70-1190-PRA0043
Product DescriptionGlidewell HT™ Implant Esthetic Abutments are prefabricated, screw-retained intraoral abutments intended to be connected directly to an endosseous implant for retention of a cemented dental prosthesis. They may be... -
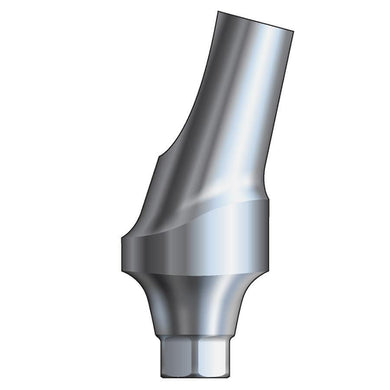
Glidewell HT™ Implant 15° Anterior Esthetic Abutment - Ø5.0 Implant
70-1190-PRA0044
Product DescriptionGlidewell HT™ Implant Esthetic Abutments are prefabricated, screw-retained intraoral abutments intended to be connected directly to an endosseous implant for retention of a cemented dental prosthesis. They may be... -
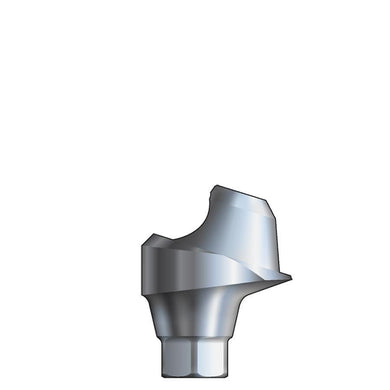
Glidewell HT™ Implant 15° Posterior Esthetic Abutment - Ø5.0 Implant
70-1190-PRA0045
Product DescriptionGlidewell HT™ Implant Esthetic Abutments are prefabricated, screw-retained intraoral abutments intended to be connected directly to an endosseous implant for retention of a cemented dental prosthesis. They may be... -
Glidewell HT™ Implant 17° Multi-Unit Abutment 2.5 mmH - Ø3.5/4.3 Implant
70-1190-PRS0001
Product DescriptionGlidewell HT™ Implant Multi-Unit Abutments are prefabricated, screw-retained intraoral abutments intended to be connected directly to endosseous implants in partially or fully edentulous patients for the retention of cast...